Student: Figure 11 in Proske & Gandevia (2012) is fixed. I used plurals and changed Motor Command to Motor Commands and Efference Copy to Efference Copy Signals!
Professor: Attaway. Nice quick thinking.
Student: And where do the Efference Copies go?
Professor: Actually, as many as four copies with one coming:
1) from the spinal cord and going to an oculomotor nucleus,
2) from the spinal cord and going via the spinocerebellar tract to the cerebellum,
3) from the frontal cortex, going to the basal ganglia, then on to the cerebellum, and
4) from the frontal cortex and going via the pontine nuclei to the cerebellum.
Professor: A more important question is, “What is the sensory signals?”
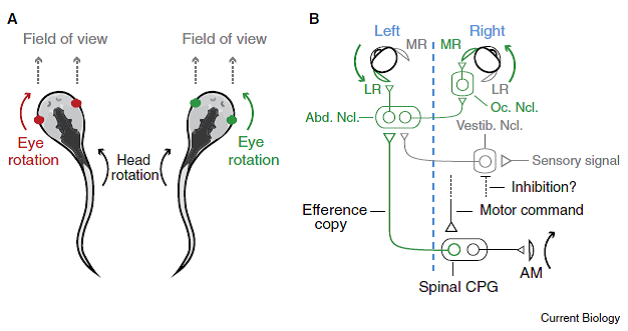
First, for the eye, vestibular signals come from ocular reflexes, for example, in a tadpole as it undulates using tail muscles to swim. And, Lambert et al. (2012) studied fictive swimming of tadpoles, and their results have been redrawn in Figure 1 in a very supportive comment by Bagnall & McLean (2012) that is shown below:
“Figure 1. Counter-rotation of the eyes during swimming movements is now explained by a simple wiring diagram involving the spinal cord.
- “When Xenopus tadpoles swim, the body undulates back and forth, causing the head to rotate to the left and right. These movements are associated with eye rotations in the opposite direction, which maintains a relatively fixed field of view in front of the animal.
- “The out-going (‘efferent’) motor command reaches the spinal cord and activates the spinal central pattern generator (CPG), initiating a body bend via axial muscles (AM) in the tail that rotates the head to the right.
- “Spinal interneurons relay [copies of ]this signal (efference copy) across the body back to the ocular motor pools, which generate bilateral eye rotation to the left.
- “Within the abducens nucleus (Abn. Ncl.) are motoneurons that drive lateral rectus (LR) muscle activation and
- “interneurons that activate motoneurons in the ocular nucleus (Oc. Ncl.), which in turn drive medial rectus (MR) muscle activation.
- “Sensory signals arriving via the vestibular nucleus (Vestib. Ncl.) target the same nuclei, but are presumably inhibited by an as yet unknown pathway.
“For simplicity, only the circuit driving leftward eye movements is illustrated (green in A). A blue dashed line separates the left and right sides of the body.” [Emphases and formatting added]
Professor: From this figure and its legend modified from Figure 5 D in Lambert et al. (2012), the spinal interneurons are the carrier of an efference copies that bypass the vestibular nucleus to drive the ocular muscles. But when the re-afferent inner-ear signals for rotation do arrive at the vestibular nucleus, the output of the reflex is somehow suppressed by active medial and lateral rectus ocular muscles.
Bagnall & McLean (2012) also references studies of Roy & Cullen (2004) that demonstrated movement “suppression occurs only for expected ‘proprioceptive’ sensory input from neck muscles matches expected neck motion”.
Student: That is a fascinating oculomotor over-ride system. Let’s get back to locomotion and limb muscle feedback suppression circuitry.
Professor: Limb muscle feedback comparison starts out with spinal interneurons, too.
Student: How is the feedback from the motor system separate what is happening in muscle activity from what external forces changes locomotion? Like when you step in a small hole in the ground?
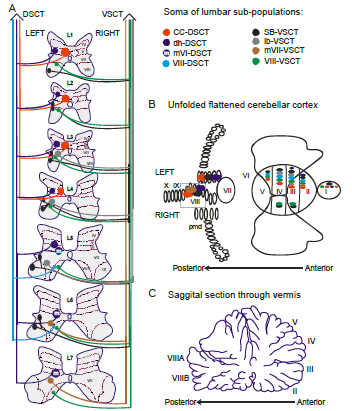
Professor: Very interesting example. Stecina et al. (2013) have written an intriguing review entitled, “Information to cerebellum on spinal motor networks mediated by the dorsal spinocerebellar tract”. Much of their information is from the cat and shown below for the dorsal spinocerebellar tract (DSCT) and the ventral spinocerebellar tract (VSCT) in their Figure 2:
“Fig. 1 Putative subpopulations of feline DSCT and VSCT cells in the lumbar region.
A. The location of the somas of feline spinocerebellar tract cells (colored regions) between the first and seventh lumbar spinal segments is illustrated schematically. Dotted lines and Roman numerals indicate an approximate outline of the Rexed laminae for reference. The colors indicate the various subpopulations;
- of the DSCT:
- Clarke´s column (CC-DSCT, red),
- dorsal horn (dh-DSCT, dark blue),
- medial lamina VI DSCT (mVI-DSCT, dark blue with an ’m’),
- lamina VIII (VIII -DSCT, light blue)
- and of the VSCT:
- spinal border cells (sb-VSCT, black),
- dorsolateral column (dl-VSCT, grey),
- medial lamina VII (mVII-VSCT, brown) and
- lamina VIII (VIII-VSCT, green).
B. Illustration of the cerebellar projection areas of spinocerebellar tract neurons. The unfolded and flattened cerebellar surface as in (Grant, 1962) is labelled with Roman numerals to indicate regions according to Larsell´s defined regions (Larsell, 1953).
C. Illustration of a saggital section through the vermis and the approximate location of the indicated Larsell´s lobules for reference. ” [Emphases and reformatting added]
Student: Egad! We are that complicated. Wow!
Professor: Of course! In the beginning of their article, they wrote,
- “Emerging anatomical and electrophysiological information on the putative subpopulations of DSCT and VSCT neurons suggest differentiated functions for some of the subpopulations.
- “Multiple lines of evidence support the notion that sensory input is not the only source driving DSCT neurons and overall, there is a greater similarity between DSCT and VSCT activity than previously acknowledged.
- “Indeed the majority of DSCT cells can be driven by the spinal CPGs for locomotion and scratch without phasic sensory input.
- “It thus seems natural to propose the possibility that CPG input to some of these neurons may contribute to distinguishing sensory inputs that are a consequence of the active locomotion from those resulting from perturbations in the external world.” [Emphases and reformatting added]
Student: I get it. Subpopulations of the spinocerebellar tracts can tell about that small hole in the ground and others that are efference copies can predict our expected walking muscle actions. Are there pathways for efference copy signal from the cerebral cortex that bypass the spinal cord?
Professor: I am going to discuss cortico-striatal pathways in a later post. But now what can look at the disynaptic cortico-ponto-cerebellar pathway. Sauvage et al. (2013) very recently have studied foot movements, both real and imagined.
- “Overt movement execution and motor imagery shared a common network including the premotor, parietal, and cingulate cortices, the striatum, and the cerebellum.
- “Motor imagery recruited specifically the prefrontal cortex, whereas motor execution recruited specifically the sensorimotor cortex.
- “We also found that slow movements specifically recruited frontopolar and right dorsomedian prefrontal areas bilaterally, during both execution and mental imagery, whereas fast movements strongly activated the sensorimotor cerebral cortex.
- “Finally, we noted that anterior vermis, lobules VI/VII and VIII of the cerebellum were specifically activated during fast movements, both in imagination and execution.
- “We show that the selection of the neural networks underlying voluntary movement of the foot is depending on the speed strategy and is sensitive to execution versus imagery.” [Emphases and reformatting added]
Professor: Please note that the involvement of the cerebellum was “specifically activated during fast movements, both in imagination and execution“. Yes, during imagination, too. There must be a direct route from the cerebral cortex to the anterior vermis of the cerebellum. In fact, they wrote,
“Moreover, to the light of surprising recent findings in monkeys showing that the vermis should no longer be considered as entirely isolated from the cerebral cortex (Coffman et al., 2011 [2]), we suggest that the anterior vermis contributes to computational aspects of fast commands.”
Professor: So, below is what Coffman et al., 2011 wrote:
“First, … a portion of the cerebellar vermis is a major target of projections from the cerebral cortex.
“Second, projections to lobules VB–VIIIB of the vermis originate mainly from motor areas in the frontal lobe and especially from [primary motor center], [supplementary motor area], and [dorsal and ventral cingulate motor areas].
“Third, these projections arise from regions of cerebral cortex that represent distal as well as proximal body parts.
“These results have significant implications for the involvement of the vermis [for anticipatory postural adjustments and] for movement.”
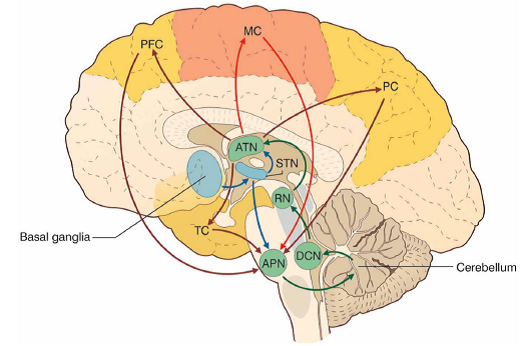
Professor: Where are these cortico-ponto-cerebellar pathway? Below is an illustration from a review article by D’Angelo & Casali:
“FIGURE 3 The cerebello-thalamo-cerebro-cortical circuits(CTCCs).
- “The figure represents schematically the bidirectional connectivity between the cerebellum and the telencephalon, in particular with the [cerebral] cortex.
- “Telencephalic projections from the cortex and basal ganglia (through the subthalamic nucleus, STN) and limbic areas are relayed to the cerebellum through the anterior pontine nuclei (APN).
- “The cerebellum in turn sends its output through the deep cerebellar nuclei (DCN), red nucleus (RN), and anterior thalamic nucleus (ATN) to various telencephalic areas including the motor cortex (MC), the prefrontal cortex (PFC), the parietal cortex (PC), and the temporal cortex (TC).
- “These connections, which are supported by anatomical and functional data, forming several bidirectional cerebello-thalamo-cerebro-cortical circuits (CTCCs).” [Emphases and reformatting added]
Student: You have found than all the same pathways that Proske & Gandevia (2012) cited for his final Figure 16.
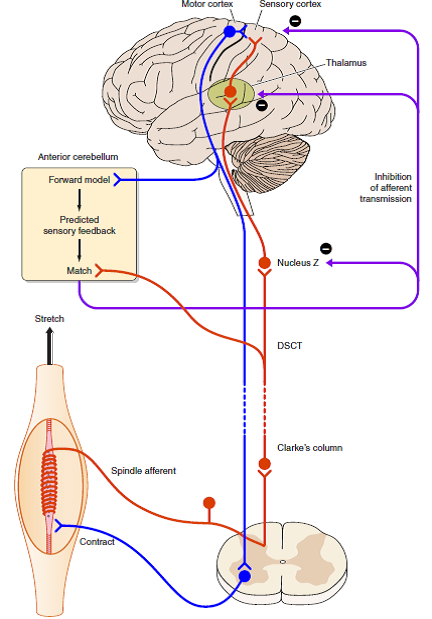
Professor: Thanks. In fact, the review of Cullen et al. (2012) citing Roy & Cullen (2004) above in the section on spinal efference copies, was also merged by Proske & Gandevia (2012) along with their “vibration observations” information and “the known central projection pathways for muscle afferents” to create their Figure 16 shown below:
“FIGURE 16. Possible mechanism (shown in diagrammatic form) for how the brain distinguishes between impulses coming from muscle spindles that are generated by muscle stretch (exafference) or by fusimotor activity (reafference).
- When the muscle is stretched, spindle impulses travel to sensory areas of the cerebral cortex via Clarke’s column, the dorsal spinocerebellar tract (DSCT), Nucleus Z, and the thalamus (shown in red).
- Collaterals of DSCT cells project to the anterior cerebellum.
- When a motor command is generated, it leads to coactivation of skeletomotor and fusimotor neurons (shown in blue).
- A copy of the motor command is sent to the anterior cerebellum where a comparison takes place between the expected spindle response based on that command and the actual signal provided by the DSCT collaterals.
- The outcome of the match is used to inhibit reafferent activity, preventing it from reaching the cerebral cortex.
- Sites of inhibition could be at Nucleus Z, the thalamus, or the cortex itself.” [Emphases and reformatting added]
Student: There it is, but that still begs the question, “What is the sense of effort?” If there is a sensory difference, as shown in their Figure 11, that is NULL, therefore no perception. Otherwise,….”
Professor: To my knowledge, they never report what the subject would report about the perception. In their studies, their instruction are to make a contraction relative to MVC or have the other arm contract to match the % MVC being experienced in the test arm.
Student: Give me a break. No verbal report. Drats. I want to control my efforts, not have them be suppressed!
Take Home: Stecina et al. (2013) identified separate DSCT and VSCT interneuron subpopulations and wrote that, “It thus seem natural to propose the possibility that CPG input to some of these neurons may contribute to distinguishing sensory inputs that are a consequence of the active locomotion from those resulting from perturbations in the external world.”
Other than that modification, the review of Proske & Gandevia (2012) is a mind-opening, clear, concise, and elegant review of “The Proprioceptive Senses: Their Roles in Signaling Body Shape, Body Position and Movement, and Muscle Force.”
Next: I want to look at the definitions of effort, exertion, and other “senses”, and then summarize how we can them as self-reports and even talk about them.
References
Bagnall MW, McLean DL. Motor Control: Spinal Circuits Help Tadpoles See learly. Current Biology, 22 (2012) R796. doi:10.1016/j.cub.2012.07.007.
Coffman KA, Dum RP, Strick PL. Cerebellar vermis is a target of projections from the motor areas in the cerebral cortex. Proc Natl Acad Sci U S A 2011;108:16068-16073.
Cullen KE, Brooks JX, Jamali M, Carriot J, Massot C. Internal models of self-motion: computations that suppress vestibular reafference in early vestibular processing. Exp Brain Res. 2011 May;210(3-4):377-88. doi: 10.1007/s00221-011-2555-9.
D’Angelo E, Casali S. Seeking a unified framework for cerebellar function and dysfunction: from circuit operations to cognition. Front Neural Circuits. 2012; 6: 116. 2013 January 10. doi: 10.3389/fncir.2012.00116.
Lambert FM, Combes D, Simmers J, Straka H. (2012). Gaze stabilization by efference copy signaling without sensory feedback during vertebrate locomotion. Current Biology, 22 (2012) 1649-1658. doi:10.1016/j.cub.2012.07.019.
Proske U, Gandevia SC. (2012) The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev 92: 1651–1697.
Roy JE, Cullen KE (2004) Dissociating self-generated from passively applied head motion: neural mechanisms in the vestibular nuclei. J Neurosci 24:2102–2111.
Sauvage C, Jissendi P, Seignan S, Manto M, Habas C. Brain areas involved in the control of speed during a motor sequence of the foot: Real movement versus mental imagery. J Neuroradiol. 2013 Feb 21. doi: 10.1016/j.neurad.2012.10.001.
Stecina K, Fedirchuk B, Hultborn H. (2013). Information to cerebellum on spinal motor networks mediated by the dorsal spinocerebellar tract. J Physiology. “Accepted Article”; doi: 10.1113/jphysiol.2012.249110