SUMMARY: This review is a collection of excerpts exploring the neurophysiological mechanisms for selective sensory filtering. That is, it investigates the gating of sensory input by the spinal cord’s dorsal horns and by the thalamic reticular nuclei.
Definitions of Sensory Gating in General
First, the review offers two similar, general definitions of sensory gating.
- “Definition: The ability of the BRAIN to suppress neuronal responses to external sensory inputs, such as auditory and visual stimuli.
- “[Note that sensory] filtering (or gating) allows humans to block out irrelevant, meaningless, or redundant stimuli.
- “Other names: Startle Gatings, Acoustic; … Gating, Sensory; Gating, Sensorimotor; … Filtering, Sensory; … [Filtering,] Sensorimotor….”
“[Sometime Sensory Gating is simplified to] ION CHANNEL GATING
- Definition: The opening and closing of ion channels due to a stimulus.
- “The stimulus can be a change in membrane potential (voltage-gated), drugs or chemical transmitters (ligand-gated), or a mechanical deformation.”
- “Gating is thought to involve conformational changes of the ion channel which alters selective permeability.”
- Definition: “A term that refers to the ability to filter (that is, to gate) sensory stimuli [input]. …
- “[Sometimes,] sensorimotor gating is taken to refer to the gating of sensory input as well as the suppression of inappropriate responses.”
- [reformatting and emphasis added]
In summary, sensory gating, or more comprehensively, sensorimotor gating, is the ability to filter input of sensory stimuli and prevent unwanted responses.
Locations Of Sensoimotor Gate Control
Gate control occurs both in the spinal cord and in the thalamus.
- Gate Control in the Spinal Cord
- The following is from the Melzack and Wall (1965) article entitled “Gate control theory of pain:”
- “Stimulation of the skin evokes nerve impulses that are transmitted ·to three spinal cord systems:
- the cells of the substantia gelatinosa in the dorsal horn,
- the dorsal-column fibers that project toward the brain, and
- the first central transmission (T) cells in the dorsal horn.
- “We propose that
- (i) the substantia gelatinosa functions as a gate control system that modulates the afferent patterns before they influence the T cells;
- (ii) the afferent patterns in the dorsal column system act, in part at least, as a central control trigger which activates selective brain processes that influence the modulating properties of the gate control system; and
- (iii) the T cells activate neural mechanisms which comprise the action system responsible for response and perception. Our theory proposes that pain phenomena are determined by interactions among these three systems.” [reformatting and emphasis added]
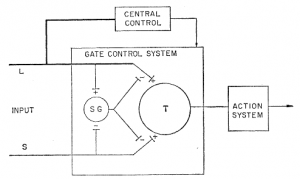
Fig. 4 in the paper by Melzack & Hall showed a “schematic diagram of the gate control theory of pain mechanisms:
“L, the large-diameter fibers; S, the small-diameter fibers.”
Furthermore, in their article, the authors stated that,
- “The fibers project to the substantia gelatinosa (SG) and first central transmission (T) cells.
- “The inhibitory effect exerted by SG on the afferent fiber terminals is increased by activity in L fibers and decreased by activity in S fibers.
- “The central control trigger is represented by a line running from the large-fiber system to the central control mechanisms; these mechanisms, in turn, project back to the gate control system.
- “The T cells project to the entry cells of the action system.
- “+, Excitation; -, inhibition”
At the time, this 1984 contribution by Melzack & Hall created quite ‘a stir’ amongst pain researchers. Its main points have been challenged, but remain almost intact.
- Minor changes have been made.
For example, the study of Duan & Cheng et al. (2014) expanded and revised details for the activities of interneurons, and was described for the lay reader by Solis (2014). He stated that,
- “Somatostatin (SOM)-positive excitatory neurons in the spinal cord are key conduits for mechanical pain, according to a report published online November 20 in Cell.
- “In the study, Qiufu Ma, in collaboration with Martyn Goulding [and their co-investigators], found that
- ablating spinal cord SOM neurons in mice abolished the animals’ responses to painful mechanical stimulation,
- yet preserved responses to heat and cold.
- “SOM-ablated mice were also protected from touch allodynia after chronic inflammation or nerve injury.
- “In addition, the researchers identified
- dynorphin (Dyn)-positive inhibitory neurons that, when ablated,
- promoted painful responses to innocuous touch.”
- “The findings also help illuminate the thicket of spinal cord wiring for pain transmission, placing the neurons in a circuit diagram proposed by the gate control theory of pain 50 years ago.”
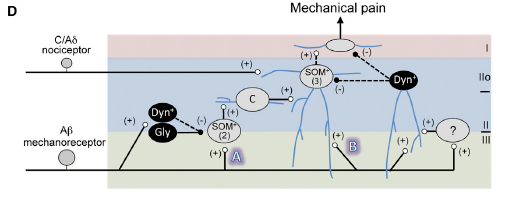
Below is Panel D in Figure 7 of the article by Duan & Cheng et al. that is a schematic showing circuitry that processes mechanical pain-related information.
The figure legend states that,
- “Vertical neurons in lamina IIo, belonging to type 3 SOM+ neurons [‘‘(3)’’], receive inputs from
- C/Aδ mechanical nociceptors, and also from
- Aβ mechanoreceptors through two pathways:
- indirect (‘‘A’’) and
- direct (‘‘B’’).
- “Pathway ‘‘A’’ is transmitted through type 2 SOM+ [‘‘(2)’’] neurons at the II-III border,
- via transient-central (‘‘C’’) cells and vertical cells in lamina IIo, and finally
- to lamina I projection neurons.
- Type 2 SOM+ neurons may include PKCγ+
- Pathway ‘‘A’’ is partly gated by Dyn+
- “Pathway ‘‘B’’ is indicated by direct Aδ inputs onto lamina IIo neurons, and is
- gated by dorsally located Dyn+ neurons that receive Aδ inputs with action potential output,
- either directly or
- via type 1 SOM+ neurons or unidentified interneurons (‘‘?’’).
- “Dashed arrows indicate that SOM+ neurons might receive direct inhibitory inputs from Dyn+ neurons, …. “
- gated by dorsally located Dyn+ neurons that receive Aδ inputs with action potential output,
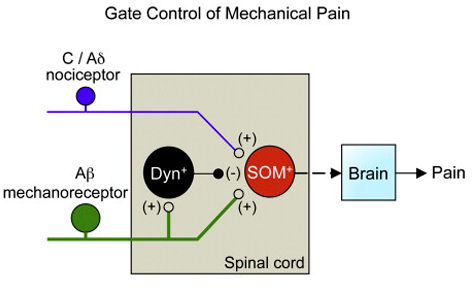
Very complicated! Solis did provide a simpler figure, perhaps overly simplified, shown below:
His figure legend stated that,
- “Genetic manipulations in the spinal cord reveal the identity of neurons transmitting and gating pain.
- “Spinal somatostatin-positive excitatory neurons
- receive input from peripheral nociceptors and
- “Aβ mechanoreceptors, which
- are in turn gated by dynorphin-expressing inhibitory neurons.
- “Image and caption [modified] from [Duan & Cheng] et al., 2014.”
In summary, sensory gate control in the spinal cord seems to occur as Melzack & Hall (1984) proposed, with minor revisions such as substituting the SOM and DYN interneurons as suggested by Duan & Cheng et al. (2014).
- Gate Control in the Thalamus
Please note the following analogy: The reticular nuclei are to the thalamus as the substantia gelatinosa is to the spinal cord.
- Crick (1984)
In the early 1980s, Sir Francis Crick wrote that,
- “The thalamus is often divided into two parts:
- the dorsal thalamus, which is the main bulk of it, and
- the ventral thalamus. … “
- “For the moment when I speak of the thalamus
- “I shall mean the dorsal thalamus.”
- “Almost all input to the cortex, with the exception of the olfactory input, passes through the thalamus.
- “For this reason it is sometimes called the gateway to the cortex. … “
- “Much of the rest of the thalamus is often referred to as the ventral thalamus [that] includes the reticular complex.
- “Again, “thalamus” means the dorsal thalamus. … “
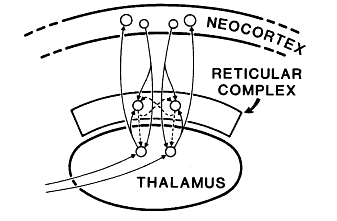
- “The reticular complex is a thin sheet of neurons, in most places only a few cells thick, which partly surrounds the (dorsal) thalamus … (see Fig. 1).
The legend above is from Figure 1 in Crick (1984) that stated,
- “The main connections of the reticular complex, [is]
- highly diagramatic and
- not at all to scale. Solid lines represent excitatory axons.
- “Dashed lines show GABAergic (inhibitory) axons.
- “Arrows represent synapses.”
Further down in his article Crick continued,
- “All axons from the thalamus to the cerebral cortex pass through it, as do all of the reverse projections from the cortex to the thalamus. … “
- It is believed that many of the axons that pass in both directions through the reticular complex give off collaterals that make excitatory synaptic contacts in it.
- If the thalamus is the gateway to the cortex, the reticular complex might be described as the guardian of the gateway. Its exact function is unknown.”
- “Not only is its position remarkable, but its structure is also unusual.
- “It consists largely (if not entirely) of neurons whose dendrites often spread rather extensively in the plane of the nucleus.
- “The size of these neurons is somewhat different in different parts of the complex.
- “Their axons, which project to the thalamus, give off rather extensive collaterals that ramify, sometimes for long distances, within the sheet of the reticular.
- “This is in marked contrast with most of the nuclei of the thalamus, the principal cells of which have few, if any, collaterals either within each nucleus or between nuclei. The nuclei of the thalamus (with the exception of the intralaminar nuclei) keep themselves to themselves.
- “The neurons of the reticular complex, on the other hand, appear to communicate extensively with each other.
- “Moreover, it is characteristic of them that they fire in long bursts at a very rapid rate.”
The next two sections provide examples of thalamic gating.
- Thalamic Gating of Neurogenic Pain
Jeanmonod et al. (1996) wrote that back, leg, and hip problems, and peripheral neuropathy experienced with diabetes are examples of the positive symptom of neurogenic pain. In the abstract of their review, they stated that,
- “Positive symptoms arise after lesions of the nervous system. …
- Stereotactic medial thalamotomies were performed on 104 patients with chronic therapy-resistant positive symptoms.
- “Peroperative recordings of 2012 single units revealed an overwhelming unresponsiveness (99%) to sensory stimuli or motor activation.
- Among these unresponsive cells, 45.1% presented a rhythmic or random bursting activity. …
- Rhythmic bursting activities had an average interburst interval of 263±46 ms corresponding to a frequency of 3.8±0.7 Hz.
- All bursts, rhythmic or random, fulfilled the extracellular criteria of low-threshold calcium spike bursts.
- “After medial thalamotomy and depending on the symptom,
- 43-67% of the patients reached a 50-100% relief
- with sparing of all neurological functions.
- “On the basis of these electrophysiological and clinical results,
- we propose a unified concept for all positive symptoms
- centred on a self-perpetuating thalamic cell membrane hyperpolarization,
- similar to the one seen in slow-wave sleep.”
They concluded that their unified concept involved three thalamic components:
- the (1) medial thalamus, the(2) lateral or specific thalamus and the (3) thalamic reticular nucleus (RTN),
- that are interconnected by inhibitory (GABAergic) reticulothalamic and excitatory thalamoreticular pathways;
- the medial thalamus has more multimodal convergent and divergent connections
- with “entres” other than the specific thalamus,
- whereas the specific thalamus has dense projections to and from restricted, unimodal and topologically organized areas.
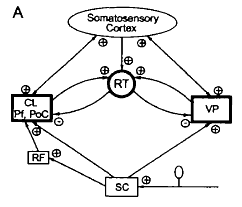
Below is Figure 7 in the article by Jeanmonod et al. showing the role of the reticular thalamic nucleus [abbreviated RT] for neurogenic pain.
The legend for Figure 3 shown above from their review shows the thalamo-cortical network for the neurons of the shoulder, elbow, and wrist/paw during locomotion. The network’s components are:
- The motor cortex (MC) is the red plate.
- All of these neurons are identified as pyramidal tract neurons (PTNs) (red arrow).
- The ventrolateral nucleus of thalamus (VL) is the blue circle and is a part of the “motor thalamus.”
- The VL receives its major input from the interposed and lateral nuclei of cerebellum (purple arrow), and also receives input from the spinal cord (green arrow).
- The VL forms the main subcortical input to the MC.
- Most neurons, whose activities are summarized here, were identified as thalamo-cortical projection neurons (TCs, blue arrow).
- TCs synapse on both PTN and interneurons of the MC (Jones, 2007).
- The motor compartment of the reticular nucleus of the thalamus (RE) is the gray plate.
- The RE is a collection of inhibitory neurons that receive inputs from TCs as well as the cortico-thalamic neurons (CT) of the motor cortical layer VI (orange arrow).
- The RE projects back to the VL, inhibiting it.
- The RE neurons, whose activities are described here, received inputs from both the MC and VL.
- Colored stars and arrows show neurons giving excitatory connections.
- Black star and arrow shows inhibitory neurons and connection.
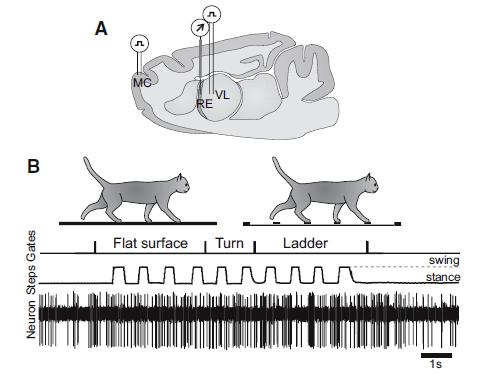
In their study that recorded RE activity, Marlinski et al. (2012b) illustrated location of brain probes and activity during walking on a flat surface and on a horizontal ladder:
The legend for Figure 1, shown above, stated the following about their experimental paradigm:.
- Panel A is a schematic drawing of a parasagittal section of the brain showing the position of chronically implanted guide tubes for recording electrodes above the RE and stimulating electrodes in the VL and MC.
- Panel B shows the locomotion tasks: walking in a high-walled, rectangular chamber along a flat surface corridor and then on the rungs of a horizontal ladder corridor.
- The trace Gates shows when the cat has passed the beginning and end of each of the chamber’s corridors.
- The trace Steps indicates the swing and stance phases of the right forelimb recorded with an electromechanic sensor.
- The trace Neuron shows discharge of a neuron from the RE.
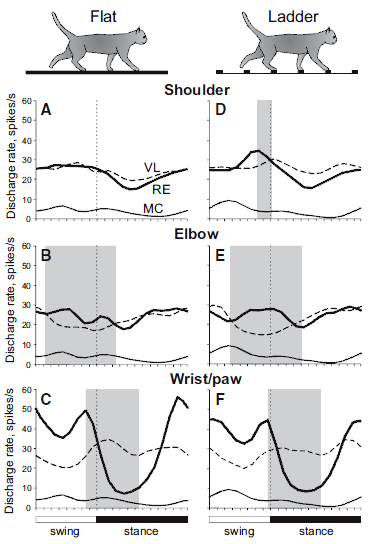
The results are summarized in the figure below, combining all three sets of animal data, which illustrate selective filtering, i.e., gating, during locomotion:
The legend for Figure 11 shown above from the review of Beloozerova et al. (2013) depicts “Differential gating” of thalamocortical signals by RE during locomotion in Panels A–F:
- “Thick lines show population activities of shoulder-, elbow-, and wrist/paw-related neurons of the RE (from Marlinski et al., 2012b); interrupted lines show activities of corresponding VL neurons (from Marlinski et al., 2012a); and
- “thin lines show the population activity of CF6 neurons of the MC (from Sirota et al., 2005).
- “Shaded are periods of the step cycle when the activities of the RE and VL were in antiphase.
- “Data on the activity of VL and CF6s neurons were obtained in exactly similar experiments, albeit conducted on different sets of cats.”
These results indicate that during locomotion, “the RTN differentially gates thalamocortical signals transmitted
- during different phases of the stride,
- in relation to different parts of the limb, and
- the type of locomotion task.”
In summary, the original drawing of Crick (1984) pointing to the pivotal role of the TRN is virtually intact, except for details added by recent studies such as Jeanmonod et al. (1996) and Beloozerova et al. (2013).
And the last, big section is on the cellular neurophysiological mechanisms for differential sensory gating by the thalamic reticular neurons.
Cellular Neurophysiological Mechanisms of Sensory (Motor) Gating
The title of an earlier study cited above provided a hint about a possible mechanism: “Low-threshold calcium spike bursts in the human thalamus” by Jeanmonod et al. (1996). Where is the location of and the mechanisms for low-threshold calcium spikes?
The answer is illustrated by Sherman, Guillery, and coworkers (Lam & Sherman, 2011; Sherman & Guillery, 2013) who looked deeper into the ideas of Crick (1984). They reported that,
- “The thalamic reticular nucleus (TRN) is organized topographically and forms
- a thin layer of GABAergic cells adjacent to the relay nuclei of the dorsal lateral aspects of the thalamus, and
- it forms a curved shell or cover that lies in the path of all the axons that connect the cortex and the thalamus in both directions.”
- “That is, the reticular cells can be recognized on the basis of where they are.
- “Any cell that lies within the borders of the thalamic reticular nucleus is a reticular cell, no matter what it looks like.
- “In general, the reticular cells are treated as a homogeneous group,
- having slender dendrites that stretch out in the plane of the reticular nucleus and
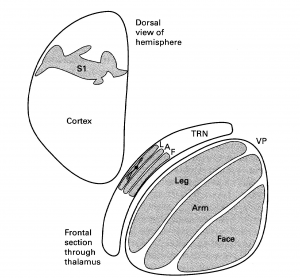
- a cell body that is also often elongated in this plane [(see layer L of the TRN in Figure 3.11 from the Sherman & Guillery book (2013) shown below):] “
The legend for Figure 3.11 stated that this is a,
- “Schema to illustrate how the parts of the body are represented in the thalamus (VP), the thalamic reticular nucleus (TRN) and the cortex of a rabbit.
- “Only one reticular cell is shown to illustrate the orientation of the reticular dendrites.
- [Abbreviations:] “A, Arm; L, Leg; T, Trunk.
- “Based on data from Crabtree (1992b).”
Again, they emphasized that,
- “Most axons connecting the thalamus and cortex in either direction pass through the TRN and innervate neurons there with collaterals;
- TRN neurons, in turn, provide strong, topographic inhibition to thalamic relay cells.”
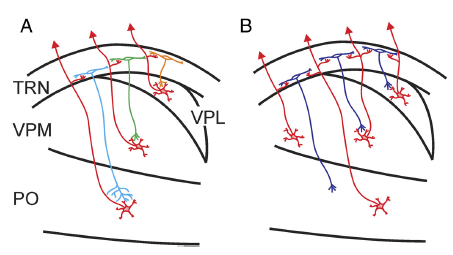
Notice as the thamocortical neurons from relay cells, shown below In figure 10 from Lam & Sherman, pass through TRN they send collaterals to reticular neurons. Consequently those activated neurons then in turn inhibit thalamic relay cells:
The legend for Figure 10 shown above, illustrated the spatial organization of the thalamoreticular pathway.
- Panel A shows a subtype of neurons that receive input from and project back to the somatosensory thalamus in a topographic manner.
- Panel B shows another subtype of neurons that receive convergent inputs from two or more thalamic nuclei and output topographically back to the thalamus.
Furthermore, they state that,
- “Most axons connecting the thalamus and cortex in both directions pass through the thalamic reticular nucleus (TRN), a thin layer of GABAergic cells adjacent to the thalamus, and innervate neurons there.
- “The TRN, therefore, is in a strategic location to regulate thalamocortical communication.
- “We recorded neurons of the somatosensory region of the TRN in a thalamocortical slice preparation and studied the spatial organization of their thalamic input using laser scanning photostimulation.
- “We show that the thalamoreticular pathway is organized topographically for most neurons.
- “The somatosensory region of the TRN can be organized into three tiers.
- “From the inner (thalamoreticular) border to the outer, in a manner roughly reciprocal to the reticulothalamic pathway, each of these tiers receives its input from one of the somatosensory relays of the thalamus—the posterior medial, ventroposterior medial, and ventroposterior lateral nuclei.
- “What is surprising is that approximately a quarter of the recorded neurons received input from multiple thalamic regions usually located in different nuclei.
- “These neurons distribute evenly throughout the thickness of the TRN.
- “Our results, therefore, suggest that there exist a subpopulation of TRN neurons that receive convergent inputs from multiple thalamic sources and engage in more complex patterns of inhibition of relay cells.
- “The findings in this study, combined with those of our recent description of corticoreticulothalamic circuitry (Lam and Sherman, 2010), suggest a complicated and versatile role for the TRN in regulating sensory inputs.
- “We offer one suggestion based on that of Crick (1984) and using his terminology: the TRN acts
- as a “searchlight” for sensory input, an externally driven searchlight that integrates cortical and subcortical inputs and then
- selectively inhibits or disinhibits thalamic relay cells, so that the appropriate information can get through the thalamus to the cortex.”
Thus, if the actions of the reticular neurons are to inhibit or disinhibit thalamic relay cells, then
the functioning of their dendritic tree could be part of the gating mechanisms.
- Like many dendritic synapses, holding a certain resting depolarization creates no disturbance and maintains the inhibition.
- That is, the present activity of the dendrite’s ionic channels thus prevents the spread of depolarization toward the cell soma and axon hillock.
- For depolarization to spread, the ionic channels have to change and
- allow a spreading active disturbance that could summate at the axon hillock and initiate an action potential or a series of action potentials along the axon.
- Remember, the discharge of a RTN will inhibit relay cells in one or several thalamic nuclei.
Sherman and Guillery (2013) provide an excellent model of channel changes and generation of action potential(s). Schematic representations of voltage-dependent ion channels underlying the conventional action potential and the low-threshold Ca2+ spike are shown below:
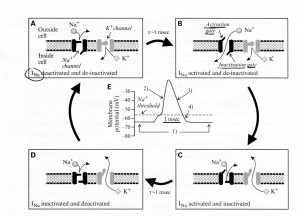
- First the legend for Figure 2.4A above shows their model for the channel events during an action potential, Panels (A)-(D), and Panel (E) shows the concurrent changes of membrane potential.
- “The Na+ channel has two voltage-dependent gates:
- an activation gate that opens at depolarized levels and closes at hyper-polarized levels, and
- an inactivation gate with the opposite voltage dependency.
- Both must be open for the inward, depolarizing Na+ current (INa) to flow.”
- “The K+ channel has a single activation gate, and when
- it opens at depolarized levels,
- an outward, hyperpolarizing K* current is activated.”
- “Panel (A) At a resting membrane potential (roughly -60 to -65 mV),
- the activation gate of the Na* channel is closed, and so it is deactivated, but
- the inactivation gate is open, and so it is de-inactivated;
- the single gate for the K+ channel is closed, and so the K+ channel is also deactivated.”
- “Panel (B) With sufficient depolarization to reach its threshold, the activation gate of the Na+ channel opens, allowing Na+ to flow into the cell;
- this depolarizes the cell, leading to the upswing of the action potential.”
- “Panel (C) After the depolarization is sustained for roughly 1 ms,
- the inactivation gate of the Na* channel closes and
- the slower K+ channel also opens;
- these combined channel actions lead to the repolarization of the cell.
- “Panel (D) Even though the initial resting potential is reached,
- the Na* channel remains inactivated because it takes
- roughly 1 ms of hyperpolarization for de-inactivation.”
- “Panel (E) Membrane voltage changes showing action potential corresponding to the events in Panels (A)-(D).”
The ability to generate LTC spikes is due to Ca2+ channels that are
- found only in a few neural tissues (thalamic cells relay cells, interneurons, reticular cells, and some other neurons)
- located on dendrites and soma, but not on axons (so LTC spikes will not go to cortical regions)
- activated by low threshold depolarization due to smaller depolarization results in action potential than Na+, and
- ready to respond by firing in either tonic mode or in burst mode.
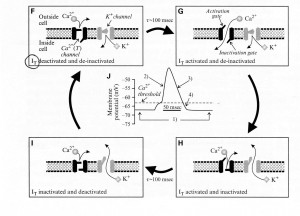
Second, see Panels (F-J) below for a schematic representation of transient (T) voltage-dependent (Ca2+) + channel underlying the low-threshold Ca2+ spike:
In Figure 2.4B above, the conventions are the same as in Panels (A)-(E);
- “Panels (F)-(I) show the channel events, and Panel (J) shows the concurrent changes of membrane potential.”
- “Note the strong qualitative similarity between the behavior of the T-type Ca2+ channel here and the Na+ channel shown in Panels (A)-(E),
- including the presence of both activation and inactivation gates with similar relative voltage dependencies.”
- “Panel (F) At a relatively hyperpolarized resting membrane potential (roughly -70 mV),
- the activation gate of the T-type Ca2+ channel is closed, but the inactivation gate is open, and so
- the T-type Ca2* channel is deactivated and de-inactivated K+ channel is also deactivated.
- “Panel (G) With sufficient depolarization to reach its threshold,
- the activation gate of the T-type Ca2+ channel opens, allowing Ca2+ to flow into the cell.
- This depolarizes the cell, providing the upswing of the low-threshold “
- “Panel (H) The inactivation gate of the T-type Ca2+ channel closes after roughly 100 ms,
- inactivating the T-type Ca2+ channel, and
- the K1” channel also opens.”
- “Panel (I) These combined actions repolarize the cell.”
- “Panel (J) Membrane voltage changes showing action potential corresponding to the events in Panels (A)-(D).”
The properties of IT and the low-threshold Ca2+ spike that shape output are shown in figure below:
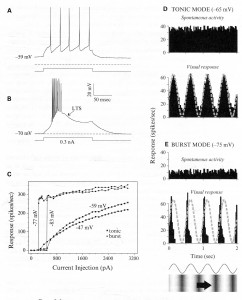
The legend for Figure 2.6 shown above, presents “examples from relay cells of the cat’s lateral geniculate nucleus recorded intracellularly,
- “either in an in vitro slice preparation (Panels A-C) or in
- “an anesthetized in vivo preparation (Panels D, E).
- “Panels (A and B) Voltage dependency of the low-threshold Responses are shown to the same depolarizing current pulse delivered intracellularly but from two different initial holding potentials.
- Panel (A) When the cell is relatively depolarized, IT is inactivated, and the cell responds in tonic mode, which is a stream of unitary action potentials to a suprathreshold stimulus.
- Panel (B) When the cell is relatively hyperpolarized, IT is de-inactivated, and the cell responds in burst mode, which involves activation of a low-threshold Ca2+ spike (LTS) with multiple action potentials (eight in this example) riding its crest.
- “Panel (C) The input-output relationship is shown for another cell.
- The abscissa is the amplitude of the depolarizing current pulse, and the ordinate is the firing frequency of the cell for the first six action potentials of the response.
- The initial holding potentials are shown, and -47 mV and -59 mV produce the tonic mode, whereas -77 mV and -83 mV produce the burst mode.”
- “Panels (D and E) Responses of a representative relay cell in the lateral geniculate nucleus of a lightly anesthetized cat to a sinusoidal grating drifted through the cell’s receptive field.
- The trace and grating at the bottom reflect the sinusoidal changes in luminance contrast with time.
- Current was injected into the cell through the recording electrode to alter the membrane potential.
- Thus in Panel (D), the current injection was adjusted so that the membrane potential without visual stimulation averaged -65 mV, promoting tonic firing, because IT is mostly inactivated at this membrane potential;
- in Panel (E), the current injection was adjusted to the more hyperpolarized level of -75 mV, permitting de-inactivation of IT and promoting burst firing.”
- “Shown are average response histograms to the visual stimulus (bottom histograms in Panels D and E) and during spontaneous activity with no visual stimulus (top histograms), plotting the mean firing rate as a function of time averaged over many epochs of that time.”
- “The sinusoidal changes in contrast as the grating moves across the receptive field are also shown as a dashed, gray curve superimposed on the responses in the lower histograms.”
In summary, the response profiles appear to be useful for feedforward regulation:
- In tonic mode, the response profile during the visual response accurate reflects the sine wave visual stimulus, and
- In burst mode, the companion response was narrowed and acted like an on-off signal indicating potentially hazardous, abrupt change.
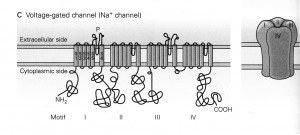
If form follows function, then, what might these channels look like? The chapter by Spiegelbaum & Koester (2013) in the latest edition of Kandel’s book, “Principles of Neural Science,” illustrates that, of the three super-families of ion channels, LTC channel has been assigned to the voltage-gated channels, that in general as below:
The legend for Panel C in their Figure 5-11 states,
- “The voltage-gated Na+ channel is formed from a single polypeptide chain that contains four homologous domains or repeats (motifs I-IV), each with six membrane-spanning a-helixes (S1-S6).
- “The S5 and S6 segments are connected by an extended strand of amino acids, the P-region, which dips into and out of the external surface of the membrane to form the selectivity filter [or gate] of the pore.”
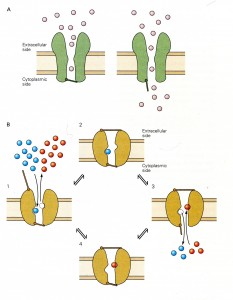
The selectivity filters, for these channels, are similar to the “P-region” gate shown above and can have either single gates or dual gates as drawn below:
The legend for their Figure 5-19 from Spiegelbaum & Koester (2013) shown above depicts the functional difference between ion channels with single gates and pumps with dual gates:
- “Panel A [Single gated Ion channels] have a continuous aqueous pathway for ion conduction across the membrane, and
- this pathway can be occluded by the closing of a [single] gate. “
- “Panel B [Dual gated ion pumps] have two gates in series that control ion flux.
- “The gates are [not necessarily opened simultaneously,] but both can close [independently] to trap one or more ions in the pore.
- “The type of pump illustrated here moves two different types of ions in opposite directions and is termed an exchanger or antiporter Ion movement is tightly linked to a cycle of opening and closing of the two gates.
- Ion movement is tightly linked to a cycle of opening and closing of the two gates.
- ([But ion transport can also be illustrated as having single ion coupled with ATP splitting that actively powers the conformational change.]
- “(1) When the external gate is open, one type of ion (red) leaves …. This triggers a conformational change, causing the external gate to shut, thereby trapping the incoming ion.
- “(2) A second conformational change then causes the internal gate to open, [expelling] the trapped ion to leave….
- “(3) A further conformational change opens the external gate, allowing the cycle to continue.
- “(4) With each cycle, one type of ion is transported from the outside to the inside of the cell….
- “By coupling the movements of ions [to an energy supply,] an exchanger can use the energy stored … to actively transport [ions] against its concentration gradient [and depolarize cell walls.]”
Summary
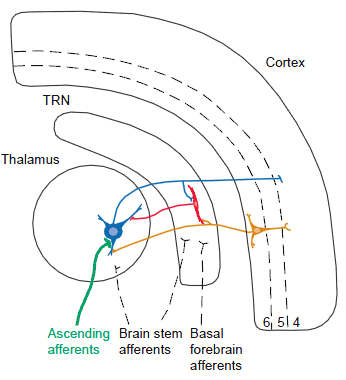
The figure below from a review by Guillery et al. (1997), that will organize this summary.
The legend for Fig. 1 in the review of Guillery et al. (1997), states that,
- “The major connections between thalamic relay cells (blue), [the]
- cells of the thalamic reticular nucleus (TRN; red) and the
- cerebral cortex (orange).
- Cortical layers 4, 5 and 6 are indicated by numbers.”
And in the text of the review, they continue:
- “THE THALAMIC RETICULAR NUCLEUS (TRN) forms an essential part of the circuits that link the thalamus to the cerebral cortex.
- “Although these circuits have been implicated in attentional mechanisms, their functional role in the transmission of information through the thalamus to the cerebral cortex is [not yet clearly defined].
- “The reticular nucleus lies like a shield between thalamus and cortex (Fig. 1 [shown above]), so that all of the fibers passing either way between thalamus and cortex must go through the TRN.”
- “This gives the nucleus the structure of a sieve rather than a real shield.
- “Many of the fibers that go through the TRN give off side branches to supply excitatory, glutamatergic synapses to the cells of TRN, and these cells, in turn, send inhibitory, GABAergic fibers back to the thalamus.
- “The basic circuit, shown in Fig. 1, can be seen as crucial in the control of messages reaching the cerebral cortex.”
To summarize, coming almost full circle, new details can be added to the quote by Crick, :
- “If the thalamus is the gateway to the cortex,
- the reticular complex might be described as the guardian of the gateway.
- Its exact function is unknown.”
Recently, its exact function has become a bit more known, and the RTN is to filter outgoing activity by thalamic neurons by using their dendritic inhibition and de-inhibition to thereby block or allow neural transmission to cortical regions. For example, de-inhibition can allow pleasant visual and auditory sensations and a massive inhibition allows sleep to happen.
Conclusion
By means of a mosaic of TRN dendritic and soma ionic channels, sensory gating is performed that shapes output AP production.
- More accurately the reticular thalamic nucleus (RTN) selectively filters the activity of the neurons passing either way between thalamus and cortex.
- Practically all fibers pass through the TRN whose primary function is to only allow passage of salient sensory input.
- Mechanistically, the TRN is a sieve surrounding dorsal thalamus cells and its neurons inhibit the relay cells of the other thalamic nuclei.
- The RTN filtering of outgoing activity of thalamic neurons by utilizing dendritic inhibition to block or dendritic de-inhibition when ‘directed’ to release neural transmission to cortical regions.
- Increased activity of Ca2+ channels on dendrites and also soma generate LTC spikes that summate in the neuron’s axon hillock to propagate these inhibitory action potentials.
References
Beloozerova IN, Stout EE, Sirota MG. (2013) Distinct thalamo-cortical controls for shoulder, elbow, and wrist during locomotion. Fronters Comput Neurosci 7: Article 62, 1-21. doi: 10.3389/fncom.2013.00062.
Crabtree JW. (1992) The somatotopic organization within the rabbit’s thalamic reticular nucleus. Eur J Neurosci 4: 1343–1351.
Crick F. (1984) Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci USA 81:4586–4590.
Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, Krashes M, Knowlton W, Velasquez T, Ren X, Ross SE, Lowell BB, Wang Y, Goulding M, Ma Q (2014) Identification of Spinal Circuits Transmitting and Gating Mechanical Pain. Cell. 159:1417-Epub. Doi: 10.1016/j.cell.2014.11.003
Guillery RW, Feig SL, Lozsa´di DA. (1998) Paying attention to the thalamic reticular nucleus. Trends Neurosci 21:28–32.
Ion Channel Gating. http://www.reference.md/files/D015/mD015640.html.
Jeanmonod J, Magnin M, Morel A. (1996) Low-threshold calcium spike bursts in the human thalamus: Common physiopathology for sensory, motor and limbic positive symptoms. Brain 119: 363-375.
Jones, EG. (2007). The Thalamus. Cambridge: Cambridge University Press.
Kandel ER, ed. (2013) Principles of Neural Science, fifth edition. New York: McGraw Hill Medical.
Lam YW, Sherman SM (2010) Functional organization of the somatosensory cortical layer 6 feedback to the thalamus. Cereb Cortex 20:13–24. doi:10.1093/cercor/bhp077.
Lam Y-W, Sherman SM. (2011) Functional organization of the thalamic input to the thalamic reticular nucleus. J Neuroscience 31: 6791– 6799. doi:10.1523/jneurosci.3073-10.2011.
Marlinski V, Nilaweera WU, Zelenin PV, Sirota MG, Beloozerova IN. (2012a) Signals from the ventrolateral thalamus to the motor cortex during locomotion. J Neurophysiol 107: 455–472.
Marlinski et al. 2012b Marlinski,V.,Sirota,M. G.,andBeloozerova, I. N. (2012b). Differential gating of thalamo-cortical signals by reticular nucleus of thalamus during locomotion. J. Neurosci. 32, 15823–15836.
Melzack R, Wall PD. (1965). Pain mechanisms: a new theory. Science 150: 971–979.
Sensory Gating. http://www.reference.md/files/D055/mD055139.html.
Sherman SM, Guillery RW. (2013) Functional connections of cortical areas: A new view from the Thalamus. Cambridge, Mass: The MIT Press,
Siegelbaum SA, Koester J. (2013) The cells of the nervous system. In: Principles of Neural Science, fifth edition. New York: McGraw Hill Medical, pp 100-125.
Sirota M G, Swadlow HA, Beloozerova IN. (2005). Three channels of corticothalamic communication during locomotion. J Neurosci 25, 5915–5925.
Solis M. (2014) Gate Keepers: Study identifies spinal cord circuit for mechanical pain somatostatin- and dynorphin-containing neurons process signals from incoming afferents. http://www.painresearchforum.org/news/48180-gate-keepers-study-identifies-spinal-cord-circuit-mechanical-pain.
Winn P, ed. (2003) Sensory gating. Dictionary of Biological Psychology, p 1507. https://books.google.com/books?id=OEMSWCeeSPYC&dq=sensory+gating+dictionary&source=gbs_navlinks_s.